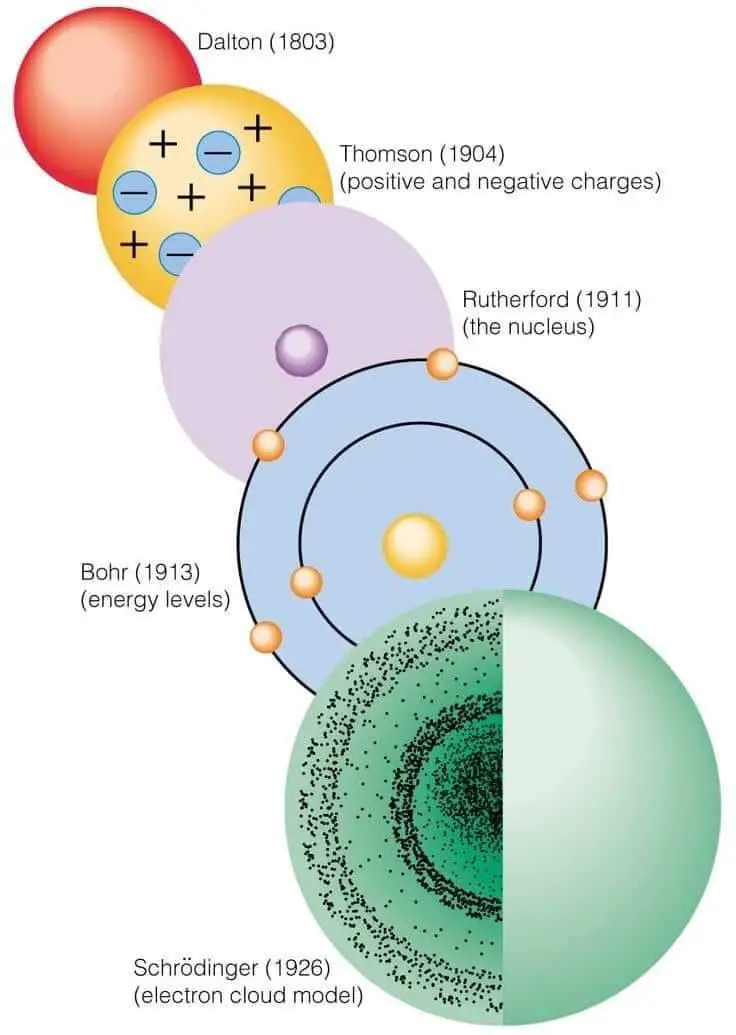
The journey to understand the atom, the fundamental building block of matter, has been a long and fascinating one, marked by significant milestones and groundbreaking discoveries. This article traces the history of atomic theory, highlighting the contributions of John Dalton, Ernest Rutherford, Niels Bohr, and Erwin Schrödinger.
John Dalton: The Revival of the Atomic Concept
In the early 19th century, John Dalton, an English chemist, and physicist, revitalized the ancient concept of the atom with his atomic theory. Dalton proposed that all matter is composed of tiny, indivisible particles called atoms. According to Dalton, atoms of a given element are identical in mass and properties, while atoms of different elements differ in these respects. He also introduced the idea that chemical reactions involve the rearrangement of atoms, which combine in fixed, whole-number ratios. Dalton's theory provided a systematic explanation for the laws of conservation of mass, definite proportions, and multiple proportions, laying the groundwork for modern chemistry.
Ernest Rutherford: The Nuclear Model of the Atom
At the dawn of the 20th century, the atomic model underwent a radical transformation thanks to the work of Ernest Rutherford, a New Zealand-born physicist. In 1909, Rutherford and his colleagues conducted the famous gold foil experiment, in which they bombarded a thin sheet of gold with alpha particles. They observed that most particles passed through the foil, but some were deflected at large angles. This unexpected result led Rutherford to propose a new atomic model in 1911. He suggested that the atom consists of a small, dense, positively charged nucleus surrounded by electrons. This nuclear model overturned the previous plum pudding model, which envisioned the atom as a diffuse cloud of positive charge with embedded electrons.
Niels Bohr: Quantum Jumps and Electron Orbits
While Rutherford's model introduced the nucleus, it did not fully explain how electrons are arranged around it. Danish physicist Niels Bohr addressed this issue in 1913 by incorporating quantum theory into his atomic model. Bohr proposed that electrons orbit the nucleus in specific, quantized energy levels and that they can transition between these levels by absorbing or emitting energy in discrete amounts, called quanta. This model explained the spectral lines of hydrogen and provided a foundation for understanding atomic structure. Bohr's theory marked a significant advancement in atomic physics, merging classical and quantum ideas.
Erwin Schrödinger: Wave Mechanics and the Quantum Model
The next major leap in atomic theory came from Austrian physicist Erwin Schrödinger in the mid-1920s. Schrödinger developed wave mechanics, a formulation of quantum mechanics that describes electrons as wave-like entities. His famous wave equation, the Schrödinger equation, provides a mathematical framework for predicting the behavior of electrons in atoms. Unlike Bohr's model, which depicted electrons in fixed orbits, Schrödinger's model treats electrons as existing in probabilistic clouds called orbitals. This approach offers a more accurate and comprehensive description of atomic and molecular systems.
Conclusion: A Continuing Journey
The development of atomic theory from Dalton to Schrödinger represents a remarkable evolution of scientific thought. Each scientist built upon the work of their predecessors, refining and expanding our understanding of the atom. Dalton's indivisible atoms, Rutherford's nucleus, Bohr's quantized orbits, and Schrödinger's wave mechanics collectively form the foundation of modern atomic physics. This journey is a testament to the power of scientific inquiry and the continual quest for knowledge, which drives us ever closer to unraveling the mysteries of the natural world.
















0 Comments, latest